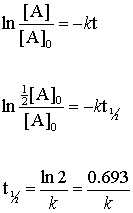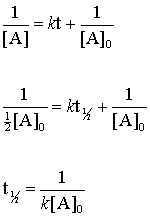half life formula for zero order reaction
Not a set value that we can calculate. T 1 2 0693 k 0693 15 10 3 min 1 46 10 2 min.
Why Is A First Order Reaction Never Complete Quora
T 12 is the half-life of.

. Equation 89 shows that the t ½ of a zero-order process is not constant but proportional to the initial concentration of drug C o and inversely proportional to the. The half-life of a first-order reaction is given as t 12 0693k. B After 5 half-lives about 38 h the.
Now replacing t with half-life t12 in the above equation. A A 0 - kt. In this instance the half-life is decreased when the original concentration is reduced to 10 M.
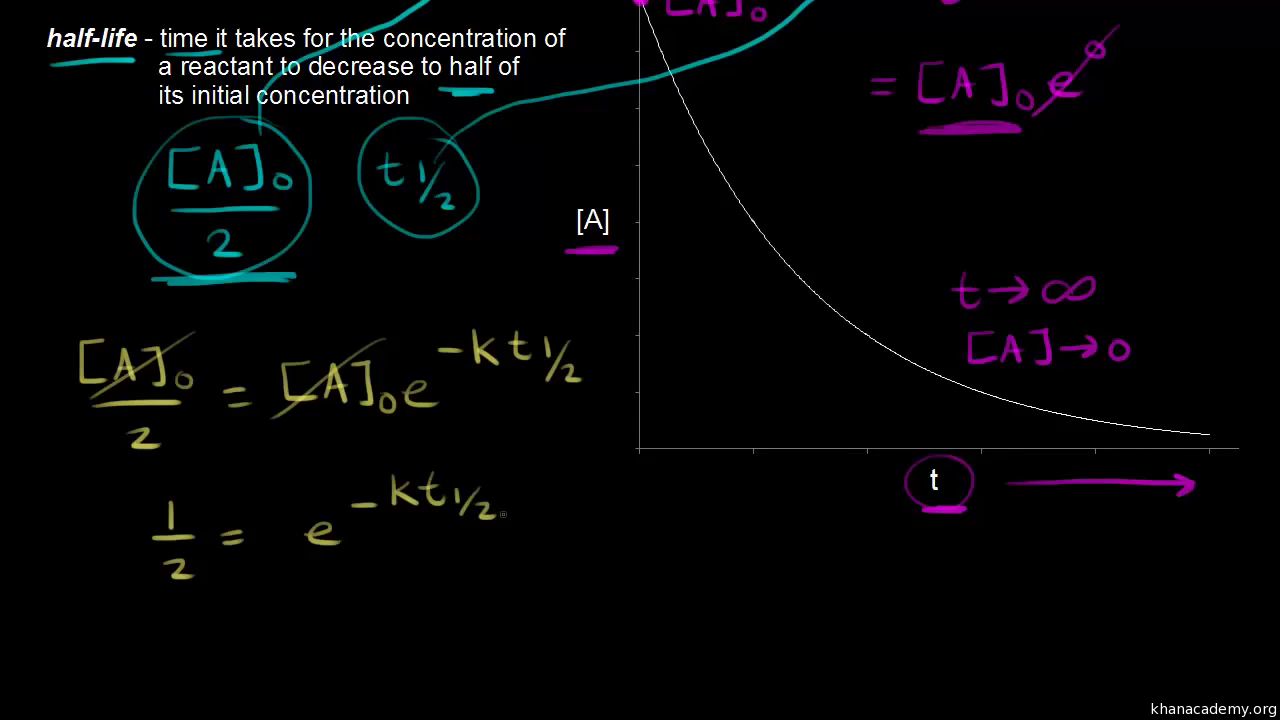
The half-life of a reaction t12. The half-life is the time required for a quantity to fall to half its initial value as measured at the beginning of the time period. If we set the time t equal to the half-life the corresponding concentration of A at this time is equal to one-half of its initial concentration.
T 1 2 1 k R 0. The half-life of a second-order reaction is given by the formula 1kR 0. Unlike a first-order reaction in a zero- or second-order reaction the half-life is dependent on the initial concentration ie.
This is an expression of the half-life of a zero-order reaction. The half-life of a Zero-th order reaction is t A0 2kHere I derive this from the Integrated Rate LawAsk me questions. If we know the integrated rate laws we can determine the half-lives for first- second- and zero-order reactions.
K R 0 R t. The order of the reaction or enough information to determine it. This is the required equation for half-life of a second-order reaction.
From the above-integrated equation we have. In some cases we need to know the initial concentration A o Substitute this information into the equation for the half life of a reaction with this order and solve for t ½. The half-life of a reaction is defined as the time required for the reactant concentration to fall to one half of its initial value.
Thus for t t 12 A t ½ A o. Since this is a zero-order reaction the half-life is dependent on the concentration. Half-Life of a Zero Order Reaction.
K t 12 12 A 0. As for all reaction orders the half-life for a zero-order reaction is inversely proportional to its rate constant. When t t ½ C C o 2 and the equation 87 becomes.
It is to be noted that the formula for the half-life of a reaction varies with the order of the reaction. I Half-Life of a Zero Order Reaction. The timescale in which there is a 50 reduction in the initial population is referred to as half-life.
What is zero order reaction with example. 1 R t 1 R 0 k t. Generally the chemical reaction carried out by a chemical catalyst is zero order.
The equation indicates that the smaller the A 0 the shorter the half-life or in other words the half-life of a zero-order reaction gets shorter as the concentration decreases. However the half-life of a zero-order reaction increases as the initial concentration increases. Substituting the value of concentration and time in the above equation we get-.
T 12 12 k A 0. It is essential to note that the half-life formula of a reaction varies with the reactions order. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t 12 R 0 2k.
The half-life of a zero-order reaction the formula is given as t 12 R 0 2k. To use this online calculator for Half Life of Zero Order Reaction enter Initial Concentration for Zero Order Reaction C0 Rate Constant of Zero Order Reaction k and hit the calculate button. From the integral form we have the following equation.
Thus it takes almost 8 h for half of the cis-platin to hydrolyze. The enzyme catalysis reaction is an example of zero order reaction with respect to the substrate. Here is how the Half Life of Zero Order Reaction calculation can be explained with given input values - 3 12000 22000.
T½ 0693 k. Rearranging this equation we have. The integrated rate constant for the zero-order reaction is given by.
We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate law as follows. It is represented by t12. A We can calculate the half-life of the reaction using Equation 453.
As for other reaction orders an equation for zero-order half-life may be derived from the integrated rate law. 12 A A 0 - k t 12. The half-life formula for various reactions is given below.
Substituting these terms into. It is the time in which the concentration of a reactant is reduced to one-half of its initial concentration. When t t12 R ½ R0.
For a zero-order reaction the integrated rate law is. For the first-order reaction the half-life is defined as t 1. Reaction B represents a.
T½ Ao 2k For a first order reaction Aproducts rate k A. And we typically use the concept of half-life to for example determine the age of ancient artifacts or predict when a radioactive sample will be safe to handle. The Half-Life of Zero Order Reaction calculator computes the half-life in nuclear decay for a zero order reaction.
The rate constant k for the reaction or enough information to determine it. And the reason for this is that most zero-order reactions either require a catalyst or occur between gases in saturated containers. Equations for half lives Determining a half life Converting a half life to a rate constant Graphical relations and half lives Equations for Half Lives For a zero order reaction Aproducts rate k.
Half-life is denoted by the symbol t 12. The new half-life is 80 seconds. Half-life t ½ or half-time is defined as the time period required for the concentration of drug to decrease by one-half.
The half-life of a reaction is referred to as t 12 unit - seconds The initial reactant concentration is referred to as R. The equation of integrated rate expression of the second-order reaction is-. T 12 A 0 2k.
The half-life equation for a zero-order reaction is t12A02k t 1 2 A 0 2 k.

Kinetics Integrated Rate Law And Half Life Expression For General Nth Order Reaction N 1 Youtube

Half Life Of A Zero Order Reaction Is 250sec T75 T100 Of The Reaction Respectively In Sec Are Edurev Neet Question
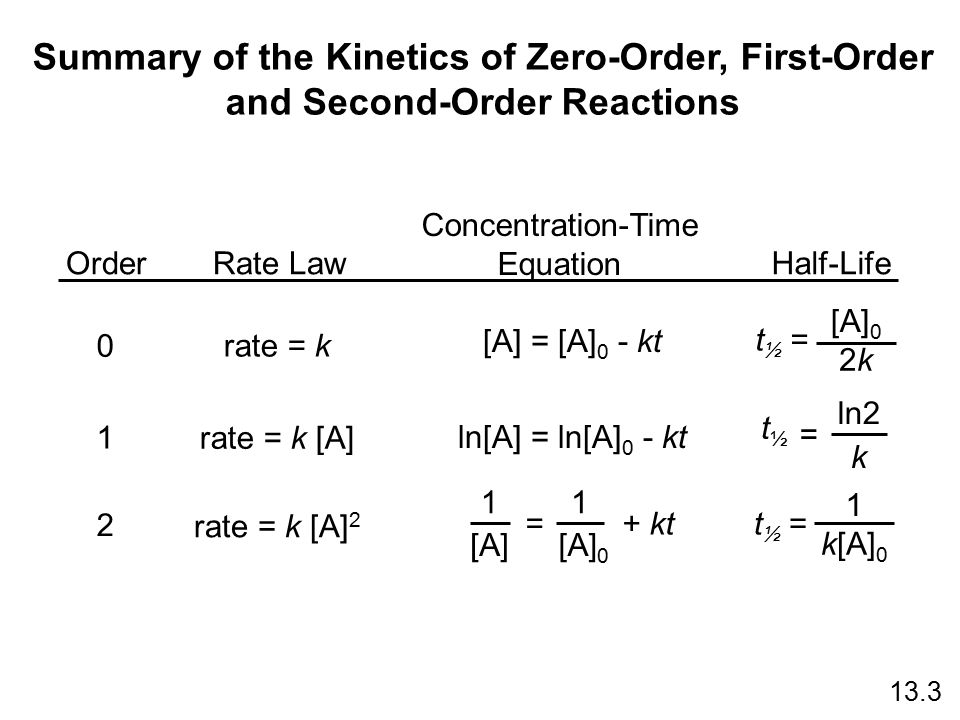
Summary Of The Kinetics Of Zero Order First Order Ppt Download

Half Life Of Zero Th 0th Order Reaction Derivation Youtube
First Order Reaction Derivation And It S Half Life Time Chemical Kinetics Chapter Youtube
Half Life Of A First Order Reaction Video Khan Academy

Half Life Expressions Chemistnate

Half Life Expressions Chemistnate

Zero Order Reactions Chemistry Class 12 Iit Jee Main Advanced Neet Aipmt Askiitians Youtube
Zero Order Reaction Definition Examples Formula

Zero Order Reactions Video Kinetics Khan Academy
Derive Half Life For Zero Order And First Order Reaction Chemistry Point

Half Life Of A First Order Reaction Kinetics Ap Chemistry Khan Academy Youtube

Integrated Rate Laws Zero First Second Order Reactions Chemical Kinetics Youtube